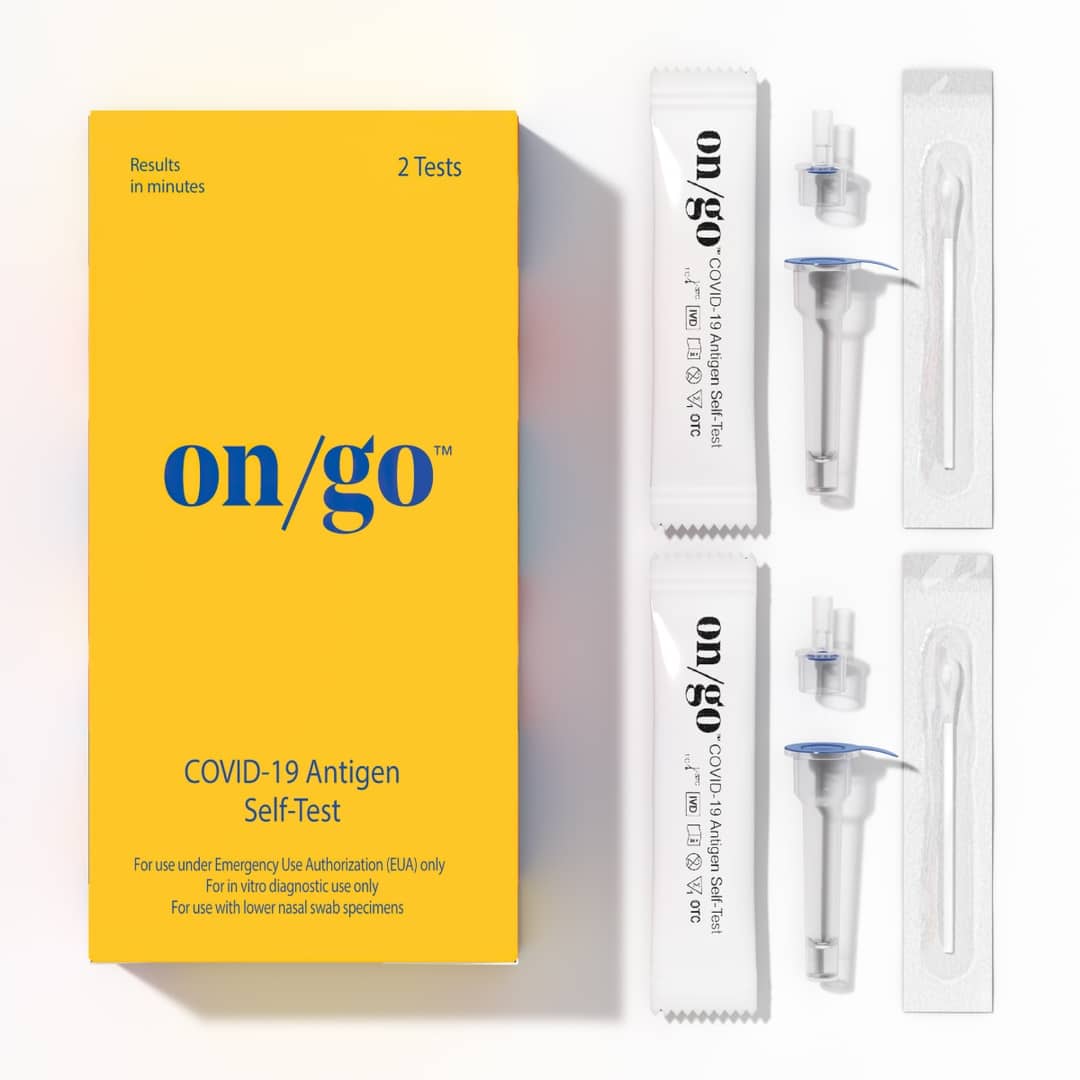
The On/Go™ COVID-19 Antigen Self-Test
The On/Go™ COVID-19 Antigen Self-Test is an over-the-counter, self-administered rapid test that delivers results in just 10 minutes, with 95% accuracy.* On/Go is proudly manufactured in the United States.
This test is authorized for non-prescription home use for individuals with symptoms of COVID-19 within the first 7 days of symptom onset. The On/Go Self-Test is also suitable for individuals without symptoms or other epidemiological reasons to suspect a COVID-19 infection when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
This test is authorized for non-prescription home use for individuals with symptoms of COVID-19 within the first 7 days of symptom onset. The On/Go Self-Test is also suitable for individuals without symptoms or other epidemiological reasons to suspect a COVID-19 infection when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.